Oxygen Number Of Valence Electrons
- O2 Number Of Valence Electrons
- Oxygen Electron Configuration And Number Of Valence Electrons
- Oxygen # Of Valence Electrons

- So chlorine has seven electrons in its valence shell and for oxygen atom, there are six electrons in its valence shell. Total valence electrons given by chlorine atoms = 7.1 = 7; There are three oxygen atoms in ClO 4-ion, Therefore. Total valence electrons given by oxygen atoms = 6. 4 = 24.
- .For atoms with LESS than 4valence electrons, they’re going to lose/give upelectrons to form positive cations.For atoms with MORE than 4valence electrons, they’re going to gain/stealelectrons to form negative anions.For atoms with 4 valence electrons, it can go either way.For atoms with 8 valence electrons, there is no change.
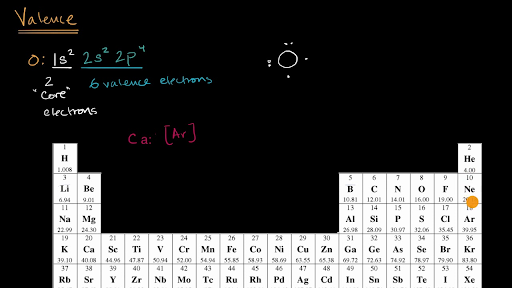
The electron configuration of an oxygen atom He 2s 2 2p 4 suggests that neutral oxygen atoms can achieve an octet of valence electrons by sharing two pairs of electrons to form an O=O double bond, as shown in the figure below. According to this Lewis structure, all of the electrons in the O.
O2 Number Of Valence Electrons
How many valence electrons does oxygen have?
1 Answer
Oxygen Electron Configuration And Number Of Valence Electrons

Oxygen has 6 valence electrons.
A way to remember this is to note that it is in column 16 of the periodic table. For the representative elements (columns 1, 2, 13-18), the digit in the units place of the column number is the same as the number of valence electrons. Elements in column 1 have one valence electrons, elements in column 13 have 3 valence electrons, etc.
Oxygen # Of Valence Electrons
The 2 electrons on the top represent the
Related questions